Science
Researchers Develop One-Pot Method for Aryne Precursors Synthesis
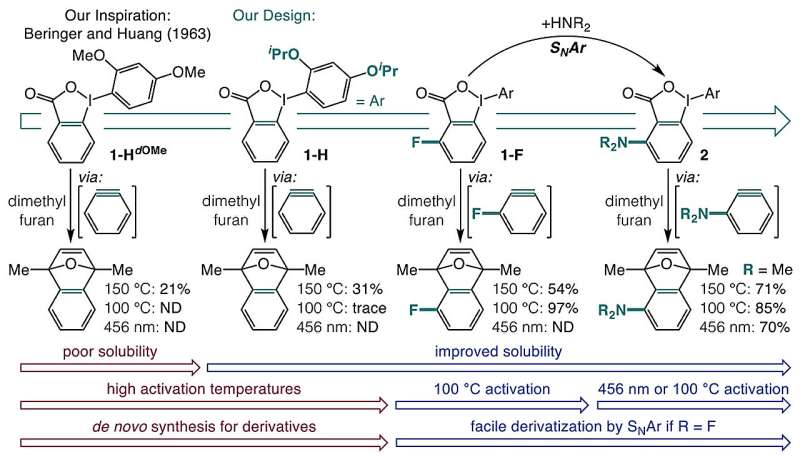
Researchers from the University of Minnesota have introduced a groundbreaking one-pot method for synthesizing blue light-responsive aryne precursors from commercially available carboxylic acids. Published in the journal Nature on November 11, 2025, this innovative approach simplifies the generation of valuable organic intermediates known as arynes, which play a significant role in drug discovery and agricultural chemistry.
Arynes are highly reactive organic compounds characterized by a triple bond within an aromatic ring. Their unique structure allows them to engage with various functional groups, making them essential for creating complex aromatic molecules. Despite their potential, arynes have not been widely adopted in synthetic chemistry due to the challenges associated with their production.
Overcoming Synthesis Challenges
Historically, the synthesis of arynes has relied on harsh chemical processes that can be unsuitable for sensitive functional groups. Traditional methods often involve using strong bases to remove protons from carbon-hydrogen bonds, followed by halide elimination. These steps can lead to unwanted reactions and make the process difficult for certain molecules. Alternative approaches, including thermally activated precursors, have proven to be highly explosive, while ultraviolet light methods frequently result in undesired side reactions.
In response to these limitations, the research team focused on using o-iodoniobenzoates as a starting point for creating aryne precursors that are both easy to synthesize and can be activated through mild heat or blue light. Their innovative one-pot synthesis effectively transforms carboxylic acids into o-iodoniobenzoate precursors.
The researchers encountered challenges with the solubility of o-iodoniobenzoates, which often led to unwanted side reactions. Through careful experimentation, they discovered that adding isopropoxy groups to the o-iodoniobenzoate structure enhanced solubility and minimized these side reactions. Additionally, they found that introducing substituents adjacent to the carboxylate group enabled the activation of aryne precursors either by heating to 100 °C or by exposure to blue light at 398 nm.
Significance of the Findings
The study revealed that heat activation is primarily influenced by a field effect. Chemical groups positioned near the carboxylate on the aromatic ring can create an electronic field that promotes decarboxylation. Meanwhile, the blue light activation excites the molecule to a triplet state, facilitating the cleavage of the aromatic ring from the iodine atom. This results in the release of carbon dioxide and the formation of arynes.
This one-pot method provides access to a diverse range of aryne precursors from readily available carboxylic acids. The researchers emphasize that their approach is compatible with various functional groups and significantly streamlines the synthesis of complex aromatic compounds, which could have far-reaching implications for pharmaceuticals and agrochemicals.
The findings of this research not only address longstanding challenges in synthetic chemistry but also open new avenues for exploring previously uncharted chemical territories. As the demand for innovative compounds grows, this method offers a promising path forward.
The full study detailing these findings is available in Nature, authored by Chris M. Seong and colleagues. For more details, refer to the published article at DOI: 10.1038/s41586-025-09830-1.
-

 Politics2 weeks ago
Politics2 weeks agoHamas Chief Stresses Disarmament Tied to Occupation’s End
-

 Science2 weeks ago
Science2 weeks agoOhio State Study Uncovers Brain Connectivity and Function Links
-

 Entertainment2 weeks ago
Entertainment2 weeks agoMegan Thee Stallion Exposes Alleged Online Attack by Bots
-

 Science4 weeks ago
Science4 weeks agoResearchers Challenge 200-Year-Old Physics Principle with Atomic Engines
-

 Entertainment2 weeks ago
Entertainment2 weeks agoPaloma Elsesser Shines at LA Event with Iconic Slicked-Back Bun
-

 Top Stories2 weeks ago
Top Stories2 weeks agoFederal Agents Detain Driver in Addison; Protests Erupt Immediately
-

 Business2 weeks ago
Business2 weeks agoMotley Fool Wealth Management Reduces Medtronic Holdings by 14.7%
-

 Business2 weeks ago
Business2 weeks agoHome Depot Slashes Prices on Halloween Favorites Up to 75%
-

 Top Stories2 weeks ago
Top Stories2 weeks agoOrioles Hire Craig Albernaz as New Manager Amid Rebuild
-

 Entertainment2 weeks ago
Entertainment2 weeks agoSyracuse Stage Delivers Lively Adaptation of ‘The 39 Steps’
-

 Top Stories2 weeks ago
Top Stories2 weeks agoWill Smith Powers Dodgers to World Series Tie with Key Homer
-

 World4 weeks ago
World4 weeks agoGlobal Military Spending: Air Forces Ranked by Budget and Capability









