Science
Researchers Slash CO2 Emissions in Fuel Production Using Iron Catalysts
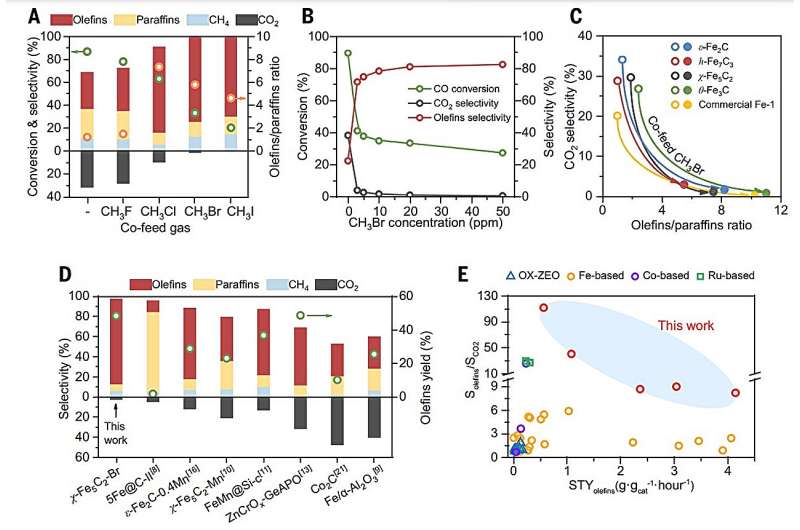
A recent study has achieved remarkable progress in reducing carbon dioxide emissions during the conversion of synthesis gas into liquid fuels. Researchers have demonstrated that by introducing trace amounts of bromomethane into the Fischer–Tropsch synthesis (FTS) process, they can decrease CO2 production by over 99%. This breakthrough was detailed in a publication in the journal Science.
Fischer–Tropsch synthesis is a critical process in the petrochemical industry, converting synthesis gas—composed of carbon monoxide (CO) and hydrogen (H2)—into liquid hydrocarbons. The research team from China found that adding bromomethane at parts-per-million levels significantly altered the reaction outcomes, making the process considerably more environmentally friendly. The typical CO2 selectivity, which ranges from 18% to 35%, was reduced to nearly zero. Concurrently, olefin selectivity surged to 85%, a dramatic improvement from the previous olefin-to-paraffin ratio of 1.3:1 to an impressive 13:1.
Despite ongoing advancements in renewable energy technologies, fossil fuels still account for over 80% of global energy consumption. To build a sustainable future, it is essential to enhance the efficiency of renewable sources while minimizing the environmental impact of fossil fuel usage. The FTS process, which has been a staple of the petrochemical industry since its inception by German chemists Franz Fischer and Hans Tropsch in the 1920s, remains critical for producing liquid fuels.
More than two-thirds of global FTS production utilizes iron-based catalysts, which yield approximately 15.70 million tons of hydrocarbons annually. These catalysts are favored for their cost-effectiveness and natural abundance but are often associated with the undesirable production of CO2. Previous efforts to minimize CO2 emissions through various modifications, such as coating iron catalysts with hydrophobic materials, yielded limited success, achieving selectivity rates below 13%.
In a significant departure from these earlier techniques, the researchers discovered a straightforward method to enhance olefin yield while curtailing CO2 emissions. By co-feeding bromomethane into the syngas during the catalytic reaction, they formed surface-bound bromine species that effectively blocked two unwanted reactions: the water-gas shift and Boudouard reactions, which produce water and CO2.
This innovative approach allowed olefins to exit the catalyst surface more readily, preventing their further hydrogenation into less desirable products. Notably, the modified catalyst displayed exceptional long-term stability, operating effectively for over 450 hours without significant degradation.
The researchers emphasize that this method can be integrated with various iron-based FTS catalysts, including commercially available formulations. By facilitating carbon-neutral conversion of coal and syngas, this advancement offers a promising link between fossil fuel chemistry and climate sustainability.
This study, led by Yi Cai and reported by Sanjukta Mondal, was closely reviewed by Robert Egan, ensuring its credibility and relevance within the scientific community. The findings not only represent a significant leap forward in reducing emissions but also promise to enhance the viability of FTS as an essential process in the ongoing transition towards greener energy technologies.
-

 Science3 weeks ago
Science3 weeks agoResearchers Challenge 200-Year-Old Physics Principle with Atomic Engines
-

 Politics1 week ago
Politics1 week agoHamas Chief Stresses Disarmament Tied to Occupation’s End
-

 Science1 week ago
Science1 week agoOhio State Study Uncovers Brain Connectivity and Function Links
-

 Top Stories1 week ago
Top Stories1 week agoFederal Agents Detain Driver in Addison; Protests Erupt Immediately
-

 Entertainment1 week ago
Entertainment1 week agoMegan Thee Stallion Exposes Alleged Online Attack by Bots
-

 Entertainment2 weeks ago
Entertainment2 weeks agoSyracuse Stage Delivers Lively Adaptation of ‘The 39 Steps’
-

 World3 weeks ago
World3 weeks agoGlobal Military Spending: Air Forces Ranked by Budget and Capability
-

 Top Stories1 week ago
Top Stories1 week agoWill Smith Powers Dodgers to World Series Tie with Key Homer
-

 Politics3 weeks ago
Politics3 weeks agoNHP Foundation Secures Land for 158 Affordable Apartments in Denver
-

 Top Stories1 week ago
Top Stories1 week agoOrioles Hire Craig Albernaz as New Manager Amid Rebuild
-

 Lifestyle1 week ago
Lifestyle1 week agoTrump’s Push to Censor National Parks Faces Growing Backlash
-

 Politics1 week ago
Politics1 week agoNFL Confirms Star-Studded Halftime Show for Super Bowl LVIII









