Health
Novo Nordisk’s Semaglutide Fails to Meet Alzheimer’s Trial Expectations

Novo Nordisk’s clinical trials evaluating semaglutide for Alzheimer’s disease have yielded disappointing results, failing to meet the anticipated efficacy benchmarks. The company, based in Copenhagen, had positioned semaglutide—a glucagon-like peptide-1 (GLP-1) receptor agonist—to potentially alter the course of this debilitating condition, but the outcomes have not supported this ambition.
The trials were closely monitored within the pharmaceutical industry, especially given the heightened focus on Alzheimer’s treatments. Semaglutide has previously shown promise in managing type 2 diabetes and obesity, which fueled hope for its application in neurodegenerative diseases. Yet, the Phase 3 trials, which involved over 1,300 participants, did not demonstrate significant cognitive improvement compared to placebo.
Investors reacted swiftly to the news, with Novo Nordisk shares dropping by approximately 10% following the announcement. This decline reflects a broader sentiment about the drug’s potential impact, which many had pegged as a revolutionary step in Alzheimer’s therapy. Analysts had projected that a successful outcome could have generated revenues exceeding $2.3 billion annually for the company.
The company’s CEO, Marcus Schindler, expressed disappointment in the results during a press briefing on October 2, 2023. He stated, “While we remain committed to exploring treatment possibilities for Alzheimer’s, these findings remind us of the complexities involved in developing effective therapies for neurodegenerative diseases.” Schindler emphasized the need for continued research and innovation in this challenging field.
The trials had sought to evaluate not only cognitive outcomes but also the overall safety profile of semaglutide in Alzheimer’s patients. Unfortunately, the lack of statistically significant results raises concerns about the viability of GLP-1 receptor agonists as a class for Alzheimer’s treatment.
Despite this setback, Novo Nordisk plans to pursue further research into the potential applications of semaglutide and similar compounds. The company remains optimistic about its other ongoing projects related to metabolic disorders and obesity management, which have already shown considerable success.
This development is part of a broader context of challenges faced by pharmaceutical companies in the race for effective Alzheimer’s treatments. With an aging global population, the demand for viable therapeutic options continues to grow, making the search for breakthroughs increasingly urgent.
As the industry reflects on these outcomes, stakeholders will closely monitor subsequent research and clinical trials. Novo Nordisk’s commitment to advancing treatment options for Alzheimer’s disease, despite this setback, highlights the ongoing complexities and uncertainties surrounding neurodegenerative research.
-
 Science1 month ago
Science1 month agoOhio State Study Uncovers Brain Connectivity and Function Links
-

 Politics1 month ago
Politics1 month agoHamas Chief Stresses Disarmament Tied to Occupation’s End
-

 Entertainment1 month ago
Entertainment1 month agoMegan Thee Stallion Exposes Alleged Online Attack by Bots
-

 Science4 weeks ago
Science4 weeks agoUniversity of Hawaiʻi Joins $25.6M AI Project for Disaster Monitoring
-

 Science2 months ago
Science2 months agoResearchers Challenge 200-Year-Old Physics Principle with Atomic Engines
-

 Entertainment1 month ago
Entertainment1 month agoPaloma Elsesser Shines at LA Event with Iconic Slicked-Back Bun
-

 World1 month ago
World1 month agoFDA Unveils Plan to Cut Drug Prices and Boost Biosimilars
-

 Top Stories1 month ago
Top Stories1 month agoFederal Agents Detain Driver in Addison; Protests Erupt Immediately
-

 Entertainment1 month ago
Entertainment1 month agoBeloved Artist and Community Leader Gloria Rosencrants Passes Away
-

 Business1 month ago
Business1 month agoMotley Fool Wealth Management Reduces Medtronic Holdings by 14.7%
-
 Science2 weeks ago
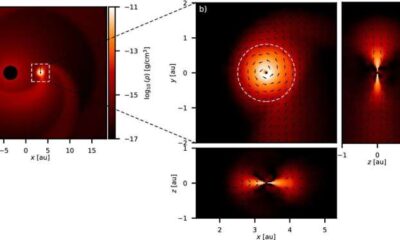
Science2 weeks agoALMA Discovers Companion Orbiting Giant Star π 1 Gruis
-

 Politics1 month ago
Politics1 month agoNHP Foundation Secures Land for 158 Affordable Apartments in Denver







