Health
Research Reveals Role of Microglia in Alzheimer’s Lipid Imbalance
Research from UT Health San Antonio and the University of California, Irvine, highlights the significant role of brain immune cells in addressing lipid imbalances associated with Alzheimer’s disease. This study challenges the longstanding focus on amyloid-beta and tau proteins, suggesting that lipid abnormalities are critical in Alzheimer’s development and progression.
More than a century ago, the first observations of Alzheimer’s disease by Alois Alzheimer noted unusual changes in brain fats, termed “lipoid granules.” Since then, research predominantly emphasized amyloid and tau, while the implications of brain lipids remained largely overlooked. The recent findings indicate that disruptions in lipid metabolism can influence amyloid accumulation and are linked to genetic factors that elevate Alzheimer’s risk.
Juan Pablo Palavicini, Ph.D., assistant professor of Cellular and Integrative Physiology at UT Health San Antonio, explained, “The brain is a unique organ. Unlike most other organs, which are rich in protein, more than half of the brain’s dry weight is made up of different kinds of lipids.” In the context of Alzheimer’s, significant lipid disruptions occur, yet research has largely neglected this area.
Microglia’s Dual Role in Lipid Regulation
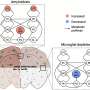
The study, published in Nature Communications, reveals how microglia, the brain’s immune cells, influence lipid changes. Depending on their manipulation, microglia can either help maintain lipid balance or exacerbate the disease. The research team, co-led by Xianlin Han, Ph.D., professor in the Department of Medicine, utilized a mouse model of Alzheimer’s to explore the impact of microglial removal.
Two methods were employed: administering a drug that nearly eliminated microglia and using genetically modified mice lacking these cells. This approach allowed the researchers to isolate the effects of microglia from other brain cells. “We wanted to understand which cells are driving these lipid changes,” Palavicini stated, noting the complexity of the interactions involved.
By comparing their findings with post-mortem brain samples from individuals with and without Alzheimer’s, they identified significant alterations in lipid patterns linked to amyloid buildup. Two specific lipid groups emerged as critical: lysophospholipids (LPC and LPE), associated with inflammation, and bis(monoacylglycero)phosphate (BMP), which regulates cellular recycling processes.
The study found that a form of BMP, enriched with arachidonic acid (AA-BMP), accumulated in proximity to amyloid plaques. Furthermore, long-term removal of microglia prevented AA-BMP buildup, suggesting that microglia play a pivotal role in these lipid changes. “BMP is still not well understood, especially in the brain,” Palavicini remarked. “Without microglia, AA-BMP levels drop, which can interfere with the brain’s cleanup processes.”
The Role of Progranulin and Other Cells
The protein progranulin, produced by both microglia and neurons, surfaced as a crucial lipid regulator. Elevated progranulin levels correlate with AA-BMP accumulation in Alzheimer’s conditions. Removing microglia resulted in decreased levels of both progranulin and AA-BMP near amyloid plaques, indicating that microglial progranulin is vital for lipid balance. “Therapies that boost progranulin could potentially restore balance and support brain health,” Palavicini suggested.
Interestingly, not all lipid changes are influenced by microglia. The study revealed that LPC and LPE levels were primarily affected by astrocytes and neurons. LPC accumulation was linked to astrocyte activation, while LPE increases correlated with oxidative stress and diminished antioxidant defenses. “This distinction helps us understand which cells to target for therapies,” Palavicini added, emphasizing the complexity of lipid regulation in Alzheimer’s.
The findings also indicated that microglia contribute to maintaining myelin, a protective sheath around neurons. The genetic removal of microglia under conditions of amyloid stress reduced myelin-related lipids. “The microglia are helping neurons, and if you remove them, neurons seem to experience more oxidative stress,” Palavicini explained.
This research paints a broader picture of Alzheimer’s disease, illustrating that it is not solely characterized by amyloid plaques and tau tangles, but also by disrupted lipid balance. Microglia, astrocytes, and neurons each play distinct roles in this process. “Understanding which cells regulate which lipids opens the door to more precise therapies,” Palavicini concluded. By addressing lipid balance alongside amyloid and tau, researchers hope to develop improved strategies for protecting neurons and potentially slowing or preventing Alzheimer’s disease.
For further details, see the study by Ziying Xu et al in Nature Communications (2025). DOI: 10.1038/s41467-025-64161-z.
-

 Science1 month ago
Science1 month agoOhio State Study Uncovers Brain Connectivity and Function Links
-

 Politics1 month ago
Politics1 month agoHamas Chief Stresses Disarmament Tied to Occupation’s End
-

 Entertainment1 month ago
Entertainment1 month agoMegan Thee Stallion Exposes Alleged Online Attack by Bots
-

 Science4 weeks ago
Science4 weeks agoUniversity of Hawaiʻi Joins $25.6M AI Project for Disaster Monitoring
-

 Science2 months ago
Science2 months agoResearchers Challenge 200-Year-Old Physics Principle with Atomic Engines
-

 Entertainment1 month ago
Entertainment1 month agoPaloma Elsesser Shines at LA Event with Iconic Slicked-Back Bun
-

 World1 month ago
World1 month agoFDA Unveils Plan to Cut Drug Prices and Boost Biosimilars
-

 Top Stories1 month ago
Top Stories1 month agoFederal Agents Detain Driver in Addison; Protests Erupt Immediately
-

 Entertainment1 month ago
Entertainment1 month agoBeloved Artist and Community Leader Gloria Rosencrants Passes Away
-

 Business1 month ago
Business1 month agoMotley Fool Wealth Management Reduces Medtronic Holdings by 14.7%
-

 Science2 weeks ago
Science2 weeks agoALMA Discovers Companion Orbiting Giant Star π 1 Gruis
-

 Politics2 months ago
Politics2 months agoNHP Foundation Secures Land for 158 Affordable Apartments in Denver









